Influence
of gibberellic acid (GA3)
on growth, chlorophyll and seed yield of summer mungbean cultivars in
Northwest of Bangladesh
Rawnak
Ara Noor-E-Ferdous1*, Md
Shariful Islam2 and Bikash
C Sarker3
1Bangladesh
Stevia and Food Industries Limited, Dhaka-1216,
Bangladesh, 2 Bangladesh
Sugarcrop Research Institute, Regional Sugarcrop Research Station,
Thakurgaon-5102, Bangladesh, 3
Department
of Agricultural Chemistry, Hajee Mohammed Danesh Science and
Technology University, Dinajpur-5200, Bangladesh.
Article
history: Received: 04.10.2020, Accepted: 24.12.2020,
Published Online:
31.12.2020
*Corresponding
author:rawnakara28@gmail.com
www.isciencepub.com
ABSTRACT
Mungbean
is an important pulse crop and its productivity is highly sensitive
to application of plant growth regulators. The
experiment was carried out to investigate the effects
of gibberellic
acid (GA3) on
growth, leaf chlorophyll and yield of summer
mungbean cultivars viz.,
V1-Binamoog-5,
V2-BARI
mung 6 and V3-Binamoog-8
along with four treatments of H1-control
(without GA3),
H2-50
ppm GA3,
H3-100
ppm GA3 and
H4-150
ppm GA3 applied
at 15, 30, 45 and 60 days after sowing (DAS). Data were recorded on
plant height, number of leaves plant-1,
leaf area plant-1,
dry root weight, root volume, number of root nodule, chlorophyll
content, proline content and seed yield. Plant height, number of
leaves plant-1,
leaf area plant-1 and
seed yield were statistically different among the cultivars and also
significantly influenced by the application of different
concentrations of GA3.
The highest plant height, number of leaves plant-1,
leaf area plant-1 and
seed yield were obtained by applying 100 ppm GA3.
The interaction effect of cultivars and different concentrations
of GA3 were
statistically significant on plant height, number of leaves plant-1,
leaf area plant-1 and
seed yield. The highest plant height, number of leaves plant-1,
leaf area plant-1 and
seed yield were obtained in Binamoog-8 by spraying 100 ppm GA3.
Therefore, it infers that foliar application of GA3at
the rate of 100 ppm and Binamoog-8 had the best
yield potentiality in Northwest of Bangladesh for profitable summer
mungbean cultivation.
Keywords:
Chlorophyll, GA3,
growth, mungbean, seed yield
To
cite this article: Noor-E-Ferdous
RA, Islam
MS and Sarker
BC. 2020. Influence
of gibberellic acid (GA3)
on growth, chlorophyll and seed yield of summer mungbean cultivars in
Northwest of Bangladesh. Intl. J. Agric. Med. Plants. 1(1): 26-35.
INTRODUCTION
Mungbean
(Vigna
radiata (L)
Wilczek) is one of the most important pulse crops of global economic
importance. It originated in the South and Southeast Asia and widely
grown in Bangladesh, India, Pakistan, Mayannmar, Thailand,
Philippinnes, China and Indonesia. Mungbean has special important as
an accommodative crop with short growing period along with
N2 fixation
in soil (Ferdous et al., 2012). Plant growth regulators (PGRs) are
being used as an aid to enhance yield of different crops (Nickell
1982, Sarker et al. 2009,
Bakhsh et al. 2011).
Gibberellins are plant hormones with a wide range of activities
including seed germination and cell elongation (Miransari and Smith
2009, Hayashi et al. 2014). Hussain et al. (2018) stated that growth
parameters showed increment with foliar spray with gibberellic acid.
It .breaks seed dormancy, stems elongation, enhances germination,
internodal length, hypocotyls growth and cell division in cambial
zone and increases the size of leaves, flowers and pods (Deotale et
al. 1998). It is well established that gibberellic acid causes a
dramatic increase in growth of mungbean. It increases dry weight
(Hore et al. 1988) as well as seed yield (Maske et al. 1998).
So, favorable conditions may be induced by applying growth regulator
like GA3 exogenously
in proper concentration at a proper time in a specific crop.
Considering the above fact, the present study was undertaken with the
response of mungbean to GA3 inrelation
to growth,
yield and qualityat
Northwest in Dinajpur region of Bangladesh for profitable
cultivation.
MATERIALS
AND METHODS
The
experiment was conducted at the Agricultural Farm of Hajee Mohammad
Danesh Science and Technology University (HSTU), Dinajpur during the
period from March to June 2011. The research site was located in
Northwest of Bangladesh, an agriculturally important region. It is
between 25.13º N latitude
and 88.23º E longitude
and at an elevation of 34.5 m above the mean sea level. The
experimental land belongs to the Himalayan Piedmont Plain,
Agro-ecological Zone (AEZ-1) and Ranishankail soil series classified
by FAO (1988). The experimental field was a medium high land having
sandy loam soil with pH 5.60. The experiment using three summer
mungbean cultivars was considered as factor A (V1-Binamoog-5,
V2-BARI
mung 6 and V3-Binamoog-8)
while factor B were fourtreatments viz,. H1-control
(without GA3),
H2-50
ppm GA3,
H3-100
ppm GA3 and
H4-150
ppm GA3.
The experiment was laid out in Randomized Complete Block Design
(RCBD) with 2 factors. Twenty combined treatments were V1
H1
, V1
H2
, V1
H3 ,
V1
H4
, V2
H1
, V2 H2
, V2
H3
, V2
H4
, V3
H1
, V3
H2
, V3 H3
and V3
H4
,respectively for the purposes.Crop management practices like
fertilizer, irrigation and pest management were done properly as and
when necessary. Three irrigations were applied where the first
pre-sowing irrigation was done at the time of lime application@ 1.0 t
ha-1and
were mixed with soil before two week of seed sowing (Ferdous 2016)
for better germination, second irrigation was done after weeding and
thinning, and third irrigation was done at flowering stage. Specific
concentration of GA3 for
experimental treatments was prepared and applied in the form of
foliar sprays at 25 and 45 DAS.
Yield
and yield contributing characteristics: Three
plants were selected randomly from each plot and plant height was
measured from base of the plant up to the tip of the main stem. Plant
heights of selected plants were taken at 15, 30, 45 and 60 days after
sowing (DAS). Leaves (trifoliate) were counted on each sampled plant
at 15, 30, 45, 60 DAS and mature stage. Three plants from each plot
were collected carefully at 15, 30, 45, 60 DAS and at mature stage so
that no root damage occurred. Root volume was measured by water
displacing methods using 20 mL measuring cylinder followed by oven
dried at 65oC
for 72 hours. The average dry root weight was calculated. The number
of nodules in the root of each collected plants were counted and
noted at 30, 45, 60 DAS and at mature stage.
Chlorophyll
content: Fresh
leaf samples from mungbean plants at the flowering stage were
collected for chlorophyll estimation. Chlorophyll content of mungbean
leaves was determined by following the method described by Arnon
(1949).
Chl-a=12.21
A663-2.81A646 (mgg-1 FW)
Chl-b=20.13
A646-6.03A663 (mgg-1 FW)
Total
carotenoid = (1000A470-
2.05×Chl-a - 114.8×Chl-b) / 245 (mg g-1 FW)
by Porra (2002).
Proline
content: Free
proline content of leaves was estimated using the acid ninhydrin
method described by Bates et al. (1973). Approximately 50 mg of fresh
leaf sample (same leaf sample for chlorophyll estimation) at
flowering stage was collected in a 2-mLEppendorf tube and extract was
prepared using 3% sulfosalicylic acid. The optical density of
solutions (sample solutions and standard solution) was measured at
520 nm wavelength using UV-visible spectrophotometer with the help of
standard curve using proline standard series solution.
Seed
yield: Seeds
obtained from each unit plot were sun-dried and weighed
carefully. The pod was collected by handpicking when full
maturity came turning brown to black in color. Seed weights of sample
plants were added to the respective unit plot yield to record the
final seed yield per plot. Seed yield was expressed as kg ha-1 after
adjusting at 10% moisture level.
Statistical
analyses: The
obtained data on different parameters were statistically analyzed
using the MSTAT-C program. The treatment means were compared by Least
Significant Difference (LSD) followed by Duncan’s Multiple
Range (DMRT) Test (Gomez and Gomez, 1984).
RESULTS
AND DISCUSSION
Plant
height: Plant
height increased gradually with the advancement of the growth stages
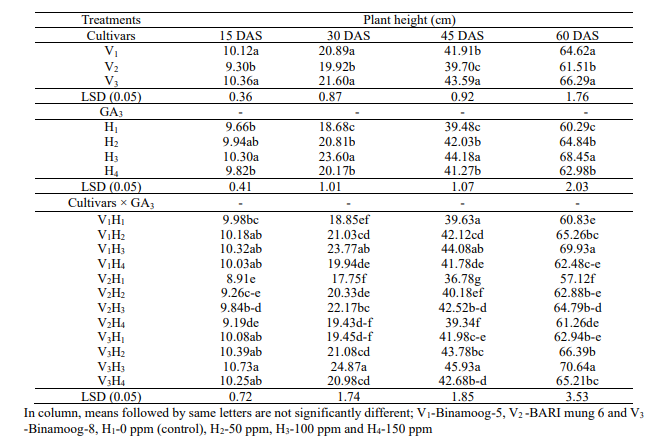
(15 -60 DAS) of the plants in all the cultivars (Table 1).
Significant variations (P<0.05)
were observed on plant height among the cultivars. The highest plant
heights (10.36, 21.60, 43.59 and 66.29 cm) were observed in
V3 (Binamoog-8)
and the lowest plant height were observed in 9.30, 19.92, 39.70 and
61.50 cm in V2 (BARI
mung-6) at 15, 30, 45 and 60 DAS, respectively. Plant height was
significantly influenced by different concentrations of GA3 at
all growth stages of mungbean (Table 1). Applying 100 ppm GA3 had
the significant effect to stimulate more cell divisions in increasing
height at 30, 45, 60 DAS and at maturity stages except 15 DAS, and
the lowest dry root weight was observed in control. The interaction
effect of cultivars and different concentrations of GA3 were
also statistically significant at different days after sowing (Table
1). The highest (10.73, 24.87, 45.93 and 70.68 cm) and the lowest
(8.91, 17.75, 36.78 and 57.12 cm) plant heights were obtained in
V3H3 (Binamoog-8
× 100 ppm GA3)
and V2H1 (BARI
mung 6 × with no GA3 application)
treatments at 15, 30, 45 and 60 DAS, respectively. From theabove
observation, it was found that plant height was increased with the
100ppm GA3in
Binamoog-8 variety. It is observed that GA3 caused
remarkably increase of plant height of' BARI mung 2 (Haque 2001). He
reported that 100 mg L-1 of
GA3 was
more effective in stem elongation in mungbean.
Table
1. Effect of
GA3 on plant height of summer mungbean and their interactions
Number
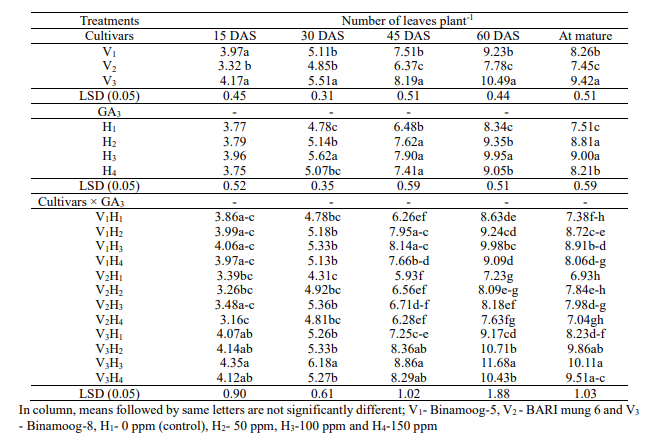
of leaves per plant:
Number of leaves plant-1 differed significantly (P<0.05)
among the cultivars at different days after sowing. The highest
number of leaves plant-1 (4.17, 5.51, 8.19, 10.49 and 9.42) was
recorded in the Binamoog-8 (V3) which were statistically different
among the cultivars and the lowest number of leaves plant-1 (3.32,
4.84, 6.37, 7.78 and 7.4) were in BARI mung 6 (V2) variety at all
stages, respectively (Table2). Significantly the highest number of
leaves (3.96, 5.62, 7.90, 9.95 and 9.00) was found spraying 100 ppm
(H3) GA3 due to its biochemical activities. The lowest number of
leaves (4.78, 6.48, 8.34 and 7.51) was observed in control plants at
30, 45, 60 DAS and mature stages, respectively except 15 DAS.
Interaction effect of cultivars and different concentrations of
GA3 was found in Table 2. A significance different was found
with the interaction effect among the cultivars and different
concentrations of GA3. The highest number of leaves plant-1was
observed in V3H3during the entire growth stages. The lowest number of
leaves plant-1 (3.16) was recorded in V2H4 at 15 DAS but
the lowest number of leaves plant-1was found during whole growing
period. Similar results was observed in Sarkar et al. (2002), who
found that GA3 using 100 ppm in soybean plants produced higher
number of leaves at the later stages of 60 and 80 DAS. Noor et al.
(2017) found that GA3 at 50 ppm concentration in treated french
bean plants produced higher number of leaves at the later stages of
48 and 58 DAS. It might be GA3 that stimulates cell enlargement
and cell division and enhances plant height, number of branches and
number of leaves in the present studies.
Table
2. Effect of
GA3 on number of leaves plant-1 of summer mungbean and
their interactions
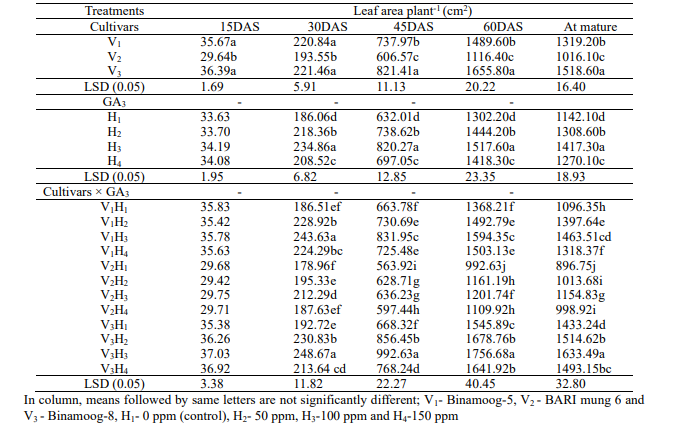
Leaf
area per plant: Effects
of GA3of
different cultivars of mungbean was significantly differed on leaf
area plant-1 at
15, 30, 45, 60 DAS and mature stage (Table 3). The highest leaf area
plant-1 was
found in V3 plants
and the lowest was observed in V2 plants
at 15 DAS. Leaf area plant-1 was
significantly influenced by the application of different
concentrations of GA3 at
all growth stages of mungbean (Table 3). Significantly the maximum
leaf area plant-1 was
observed while spraying 100 ppm GA3 at
30, 45, 60 DAS and mature stage, respectively. The lowest leaf area
plant-1 was
found spraying H1 at
30, 45, 60 DAS and mature stage, respectively. A significant
variation was found on leaf area plant-1 at
15, 30, 45, 60 DAS and at mature stages by the interaction effect
between cultivars and different concentrations of GA3 (Table
3). Significant maximum leaf area plant-1 was
observed by applying V3H3 at
30 DAS to mature stage except at 15 DAS. Similarly, the lowest leaf
area plant-1 was
obtained in V2H1at
30 DAS from mature stage. GA3 induced
higher leaf areas were reported in mungbean plant by Rahman et al.
(2018), in rice plants (Liu et al. 2012) and tomato plants (Khan et
al. 2006).
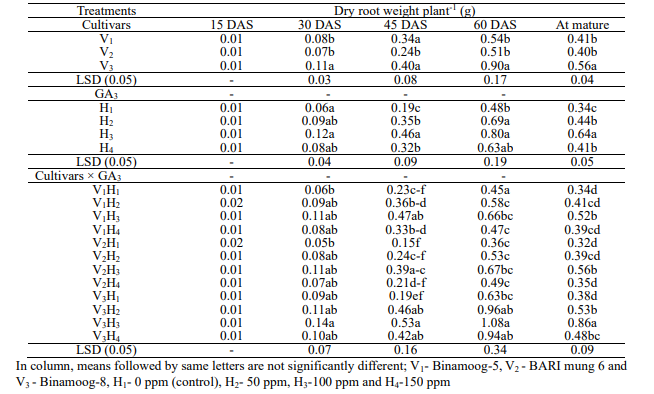
Dry
root weight per plant: There was a significant variation of
dry root weight plant-1was observed except 15 DAS (Table
4). Dry root weight was the maximum in Binamoog-8 and BARI mung 6
during the whole growth stages using different levels of GA3.
Spraying 100 ppm GA3 had the significant effect to
produce more biomass in root at all stages except 15 DAS and the
lowest dry root weight was observed in control. Dry root weight
showed significant difference among the interaction effect between
cultivars and different concentrations of GA3 at 30
DAS to mature stage except 15 DAS (Table 4). Among the interaction
effect, the highest dry root weight was observed in V3H3 (Binamoog-8
× 100 ppm GA3) of 0.14, 0.53, 1.08 and 0.86 g at 30,
45, 60 DAS and mature stage, respectively. Similarly, significantly
the lowest dry root weight was observed in V2H1 (BARI
mung 6 × without GA3) treatment of 0.05, 0.15, 0.36
and 0.32 g at 30, 45, 60 DAS and mature stages, respectively. Root
dry weight was increased with increasing with NAA concentration
(Ferdous et al. 2012) in mungbean plant and similar result was
observed in Wang and Deng (1992) in rice plant.
Table
3. Effect of GA3 on leaf area plant-1 of summer
mungbean and their interactions
Table
4. Effect of GA3 on dry root weight of summer mungbean
and their interactions
Number
of root nodule:
The giberellins are also able to regulate the legume nodules
production indicated recently. Hayashi et al., (2014) reported that
the GA3 is important at two different growth stages of nodulation
including the early stage of root colonization and the late stage of
nodule production and maturity. A significant difference in number of
root nodule was observed at all growth stages among three cultivars
of summer mungbean (Table 5). Number of root nodule was the maximum
(25.03, 49.69, 94.46 and 63.64) in V3 (Binamoog-8) and minimum
(20.81, 35.50, 79.14 and 49.53) was observed in V2 (BARI mungbean) at
30 to 60 DAS and mature stage, respectively (Table 5).Number of
nodule plant-1 differed significantly among four concentrations of
GA3 at different days after sowing (Table 5). GA3 at 100 ppm had
significant effect on Binamoog-8 producing of root nodule at 30 to 60
DAS and at mature stage while the lowest number of nodule was
observed in control. The interaction effect of cultivars and
different concentrations of GA3 were also statistically significant
at different days after sowing (Table 5). Developments of root nodule
are mainly dependent on phosphorous availability and the function of
root nodule forming bacteria. Growth regulators cause increase in
activity of root system and enhances biotic activities in the
rhizosphere. Similar findings were reported, who stated that the
foliar application of nutrients and growth regulators found to
increase in the morpho-physiological parameters, number of root
nodules plant-1 and dry weight of nodule in soybean by the foliar
application of hormones and nutrients (Raut et al. 2017, Ketki and
Thakare 2006). Foliar application of GA3 induced this effect at all
the concentration applied. The present finding was in good agreement
with Uddin (2009). The greater numbers of root nodule are beneficial
for fixing atmospheric N2 by rhizobium bacteria resulted in N supply
in soil. The present study indicated that the higher root nodulation
produced by foliar spraying of GA3 might nourish N requirement for
the mungbean plants, which might have supported N nutrition. Higher
nodule formation in summer mungbean roots applying NAA application
was reported by Ferrdous et al. (2012).
Table
5. Effect of
GA3 on number of root nodule of summer mungbean and their
interactions
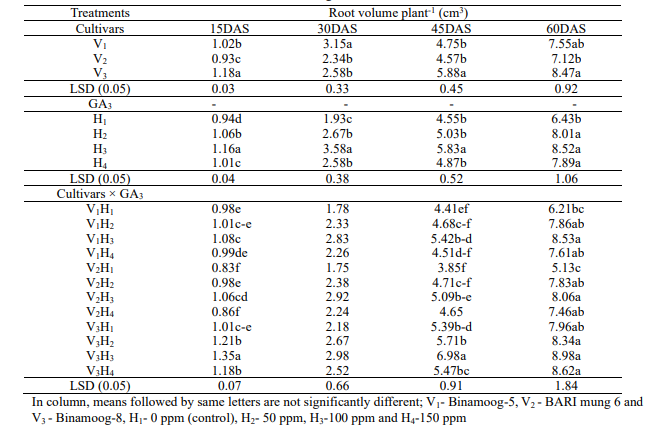
Root
volume: Root
volume was recorded from 15 to 60 DAS. Significant maximum root
volume was observed in V3 (Binamoog-8) treatment at 45 and 60
DAS (5.88 and 8.47cm3). Significant the lowest root volume was
observed in V2 (BARI mung 6) at 15to 60 DAS (1.18, 2.34, 4.57
and 7.12 cm3) treatment (Table 6). The highest root volume was
observed in H3 (1.16, 3.58, 5.83 and 8.52 cm3)at all growth
stages and the lowest root volume was observed in control at 15 to 60
DAS(0.94, 1.93, 4.55 and 6.43cm3), respectively. The interaction
effect of cultivar and different concentrations of GA3 for root
volume was significant at 15 to 60 DAS. It is clear from the results
that the highest root volume was observed in V3H3 treatment and
the lowest was observed in V2H1 at 15 DAS and 60 DAS. Ferdous et
al.(2012) revealed that NAA induced higher root volume in mungbean
plants, which conformed the present study.
Table
6. Effect of
GA3 on root volume of summer mungbean and their interactions

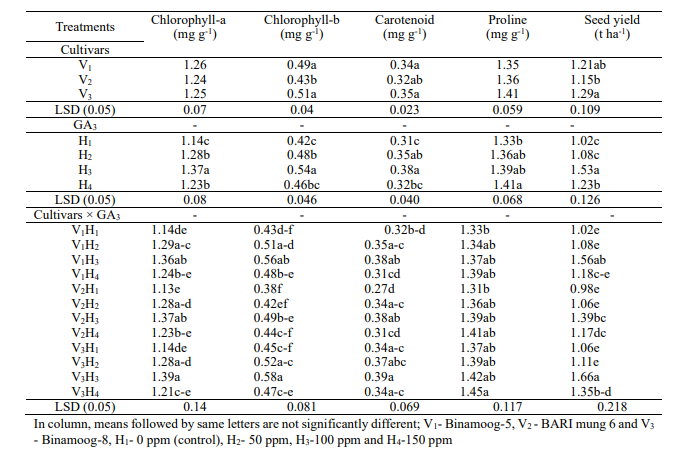
Chlorophyll
content: There
was no significant effect on chlorophyll-a content in leaves among
the cultivars but levels of GA3 (Table
7). It is noted that a significant effect was observed in the
interaction among the cultivars and levels. The highest chlorophyll-a
(1.37 mg g-1FW)
was found in with the application of 100 ppm GA3 and
the lowest (1.14 mg g-1 FW)
was observed in control (H1)
treatment. The highest amount of chlorophyll-a was obtained (1.39 mg
g-1 FW)
in V2H3 leaves
and the lowest chlorophyll-a (1.13 mg g-1 FW)
was in V2H1 leaves.
Chlorophyll-b was maximum in V3but
minimum in V2 treatment.
Considering levels of GA3,
the highest chlorophyll-b content (0.54 mg g-1 FW)
was obtained in H3 while
the lowest content was 0.42 mg g-1FWin
H1 (Control).The
highest chlorophyll-b (0.58 mg g-1 FW)
was obtained in V3H3 and
V1H3treatments,
while the lowest chlorophyll-b (0.38 mg g-1 FW)
was observed in V2H1 leaves.
Carotenoid
content: Results
in Table 7 showed that carotenoid content differed significantly
among the cultivars. Binamoog-8 (V3)
observed the highest carotenoid content (0.35 mg g-1FW),
which was statistically different from other cultivars and carotenoid
content (0.32 mg g-1FW)
was found from BARI mung 6. This variation might be due to the
GA3 effect
on different physiological activities. Carotenoid content was
significantly influenced by application of different concentrations
of GA3 (Table
7). The highest carotenoid content (0.38 mg g-1FW)
was obtained in H3,
while the lowest content (0.31 mg g-1FW)
was found in H1 treatment.
The interaction effect of cultivars and different concentrations of
GA3 was
statistically significant on carotenoid content.
Proline
content: Proline
content was non-significant among the cultivars and ranged from 1.35
to 1.41 mg g-1 FW
but was significantly influenced by the application of different
concentrations of GA3 (Table
7). The highest proline content (1.41 mg g-1 FW)
was obtained in H4 while
the lowest (1.33 mg g-1 FW)
was obtained in H3 (without
GA3).
The interaction effect of cultivars and different concentrations of
GA3 was
also statistically significant on proline content (Table 7). The
highest proline content (1.45 mg g-1 FW)
was obtained in V3H4 while
the lowest proline content (1.31 mg g-1 FW)
was observed in V2H1.
From the present investigation, important role of GA and proline was
observed when applied as foliar spraying on mungbean. Proline
application results in its rapid uptake and coupled with its
synthesis in plant, thereby increasing the endogenous level of
proline and also plays an important role for osmotic adjustment
against various stresses (Ahmed et al. 2011). In addition to the role
of osmo-protection, proline protects the enzymes, protein structure,
cell organelles and membranes by checking lipid peroxidation and
facilitates the energy supply for plant growth, and effective
quencher of ROS formed through life cycle of plant and enhanced the
activity of antioxidant enzymes. and therefore, proline increases
resistance to unfavorable climatic conditions (Ashraf and Foolad
2007).
Seed
yield: Seed yield
differed significantly among the cultivars (Table 7). Binamoog-8 (V3)
showed the highest seed yield (1.29 t ha-1),
which was statistically different from other cultivars. Seed yield
was significantly influenced by the application of different
concentrations of GA3 (Table
7). The highest seed yield (1.53 t ha-1)
was obtained in H3 (100
ppm GA3)
while the lowest (1.02 t ha-1)
was obtained in H3(without
GA3).
The interaction effect of cultivars and different concentrations of
GA3 were
found the highest seed yield (1.66 t ha-1)
in V3H3followed
by V1H3 (1.56
t ha-1).
The lowest seed yield (0.98 t ha-1)
was observed in V2H1.
The greater seed yield by applying 100 ppm GA3 due
to increased chlorophyll production and higher leaf area which might
help in enhance photosynthesis in mungbean. GA3 with
different concentrations significantly enhanced seed yield in many
crops as reported by a number of researchers (Beall et al. 1996,
Uddin1999, Sarkar et al. 2002, Tiwari et al. 2011, Alam et al. 2018,
Rahman et al. 2018, Sanjida et al. 2019).
Table
7. Effect of
GA3 on
chlorophyll, carotenoid,prolineand seed yield of summer mungbean and
their interactions
CONCLUSION
It
is concluded that gibberellic acid (GA3)
had the positive stimulatory effect on growth, chlorophyll and seed
yield production. The highest number of leaves plant-1,
leaf area plant-1along
with greater amount chlorophyll production enhanced seed yield
obtained applying 100 ppm GA3.
The interaction among the cultivars with different concentrations
of GA3 showed
statistically significant variation on the growth and yield
parameters. The highest plant height, number of leaves plant-1,
leaf area plant-1 and
seed yield were obtained in Binamoog-8 by thrice exogenous spraying
100 ppm of GA3.
It is evidently suggested that Binamoog-8, a summer munbean cultivar
in Bangladesh boosted up its yield potentiality by exogenous foliar
application of GA3 @
100 ppm. Therefore, the findings infer thatGA3 might
help in producing more summer mungbean seed yield in
Old Himalayan Piedmont Plain soil especially in Northwest of
Bangladesh for environment friendly management practices.
REFERENCES
Ahmed
CB, Magdich S, Rouina BB, Sensoy S, Boukhris M and Abdullah FB. 2011.
Exogenous proline effects on water relations and ions contents in
leaves and roots of young olive. Amino Acids. 40: 565-573.
Alam
MJ, Ahmed KS, Sultana A, Firoj SM and Hasan IM. 2018. Ensure food
security of Bangladesh: Analysis of post-harvest losses of maize and
its pest management in stored condition. Journal of Agricultural
Engineering and food Technology. 5(1): 26-32.
Arnon
DI. 1949. Copper enzymes in isolated chloroplasts and polyphenol
oxidase on Beta
vulgaris L. Plant
Physiology. 24:
1-15.
Ashraf
M and Foolad MR. 2007. Roles of glycine betaine and proline in
improving plant abiotic stress resistance. Environmental and
Experimental Botany. 59:
206-216.
Bakhsh
I, Khan HU, Khan MQ and Javaria S. 2011. Effect of naphthalene acetic
acid and phosphorus levels on the yield potential of transplanted
coarse rice. Sarhad
Journal of Agriculture. 27(2):
161-165.
Balasimha
D. 1991.Photosynthetic characteristics of cashew trees. Photosyntica.
25(3): 419-423.
Bates
LS, Waldern RP and Teare ID. 1973. Rapid determination of free
proline for water studies. Plant and Soil. 39: 205-208.
Beall
FD, Young EC and Pharis RP. 1996. Far red light stimulates internode
elongation, cell division, cell elongation and gibberellin levels in
bean. Canadian Journal of Botany. 74:
743-752.
Deotale
RD, Maske VG, Sorte NV, Chimurkar BS and Yernr AZ. 1998. Effect of
GA3 and
IAA on morphological parameters of soybean. Journal of Soils and
Crops. 8(1):
91�94.
FAO
(Food and Agriculture Organization). 1988. Land Resource Appraisal of
Bangladesh for Agricultural Development. Rep.2. Agro-ecological
regions of Bangladesh. UNDP, FAO, Rome, p. 116.
Ferdous
RANE, Islam MJ, Nahar NN, Islam MS and Sarker BC. 2012. Interactive
effects of liming and naphthalene acetic acid on growth, root
nodulation and seed yield of summer mungbean. Bangladesh Agronomy
Journal. 15(2):
37-46.
Ferdous
RANE. 2016. Studies on biochemical, physiological and molecular
aspects of summer mungbean under liming with plant growth regulators.
PhD Dissertation.Department of Agricultural Chemistry, Hajee Mohammed
Danesh Science and Technology University, Dinajpur-5200, Bangladesh
.pp.169-171.
Gomez
KA and Gomez AA. 1984. Statistical Procedures for Agricultural
Research (2nd Edition). John Wiley and Sons. New York, USA. p.680.
Haque
MM. 2001.Effect of gibberellicacid (GA3)
on growth and yield of mungbean (Vigna
radiate). M.S. Thesis.
Department of Crop Botany. Bangladesh Agricultural University,
Mymensingh. pp. 78-80.Hayashi S, Gresshoff PM, Ferguson BJ.
2014. Mechanistic action of gibberellins in legume
nodulation. Journal of Integrative Plant Biology. 56:
971–978.
Hore
JK, Paria NC and Sen SK. 1988. Effect to pre-sowing seed treatment on
germination, growth and yield of onion (Allium
cepa) var. red globe.
Harayana Journal of Horticultural Science. 179(1-2): 83-87.
Husain
AJ, Muhmood AG and Alwan AH. 2018. Interactive effect of GA3 and
prolineon nutrients status and growth parameters of pea (Pisum
sativum L.).
Indian Journal of Ecology. 45(1): 201-204.
Ketki
G and Thakare RD. 2006. Effect of foliar sprays of nutrients and
hormones on morpho physiological parameters of soybean. Journal of
Soils and Crops. 16(2): 421-428.
Khan
MMA, Gautam C, Mohammad F, Siddiqui MH, Naeem M and Khan MN. 2006.
Effect of gibberellic acid spray on performance of tomato. Turkish
Journal of Biology. 30:
11-16.
Liu
Y, Chen W, Ding Y, Wang Q, Li G and Wang S. 2012. Effect of
gibberellic acid (GA3)
and α-naphthalene acetic acid (NAA) on the growth of
unproductive tillers and the grain yield of rice (Oryza
sativa L.).
African Journal of Agricultural Research. 7(4):
534-539.
Maske
VU, Deotale RD, Sorte NV, Goramiiagar and Chore CN. 1998. Influence
of GA and NAA on growth and yield attributing parameters of soybean.
Soils and Crops. 8(I): 20-21.
Miransari
M and Smith DL. 2014. Plant hormones and seed germination.
Environmental and Experimental Botany. 99: 110-121.
Nickell
LG. 1982. Plant growth regulators (agricultural
uses). Springer-Verlag
Berlin Heidelberg, New York. p.173.
Noor
F, Hossain F and Ara U. 2017. Effects of gibberellic acid (GA3)
on growth and yield parameters of French bean (Phaseolusvulgaris L.).
Journal of the Asiatic Society of Bangladesh Science. 43(1):
49-60.
Porra
RJ. 2002. The chequered history of the development and use of
simultaneous equation for the accurate determination of chlorophylls
a and b. Photosynthesis Research. 73: 149-156.
Rahman
MM, Khan ABMMM, Hasan MM, Banu LA and Howlader MHK. 2018. Effect of
foliar application of gibberellic acid on different growth
contributing characters of mungbean. Progressive Agriculture. 29(3):
233-238.
Raut
SG, Vaidya PH, Arsud PB and Aundhakar AV. 2017. Root nodules, yield
and quality of soybean (Glycine
max L. merrill)
as influenced by foliar application of growth regulator. Journal of
Pharmacognosy and Photochemistry. 1: 130-132.
Sanjida
T, Sikdar MSI, Islam MS, Rahman MM and Alam MJ. 2019. Response of
mungbean growth and yield to GA3 rate
and time of application. Asian Journal of Crop, Soil Science and
Plant Nutrition. 1(2): 28-36.
Sarkar
PK, Haque MS and Karim MA. 2002. Growth analysis of soybean as
influenced by GA3 and
IAA and their frequency of application. Journal of Agronomy. 1:
123-126.
Sarker
BC, Roy B, Nasirullah MT, Islam MA, Sarker BC and Rahmatullah NM.
2009. Root growth, hydraulic conductance and cell wall properties of
rice root under interactive effect of growth regulator and limited
water. Journal of Agroforestry and Environment. 3(2):227-230.
Tiwari
DK, Pandey P, Giri SP and Dwivedi JL. 2011. Effect GA3 and
other plant growth regulators on hybrid rice seed production. Asian
Journal of Plant Science.10(2):
133-139.
Uddin
MH. 1999. Effect of plant growth regulators on flowering, pod set and
yield attributes in mungbean. M.S. Thesis. Department of Crop Botany,
Bangabandhu Sheikh MujiburRahman Agricultural University, Gazipur.
pp. 4-36.
Wang
SG and Dang RF. 1992. Effect of brassionosteroid (BR) on root
metabolism in rice. Journal of Southwest Agricultural University.
14(2): 177-181.





